
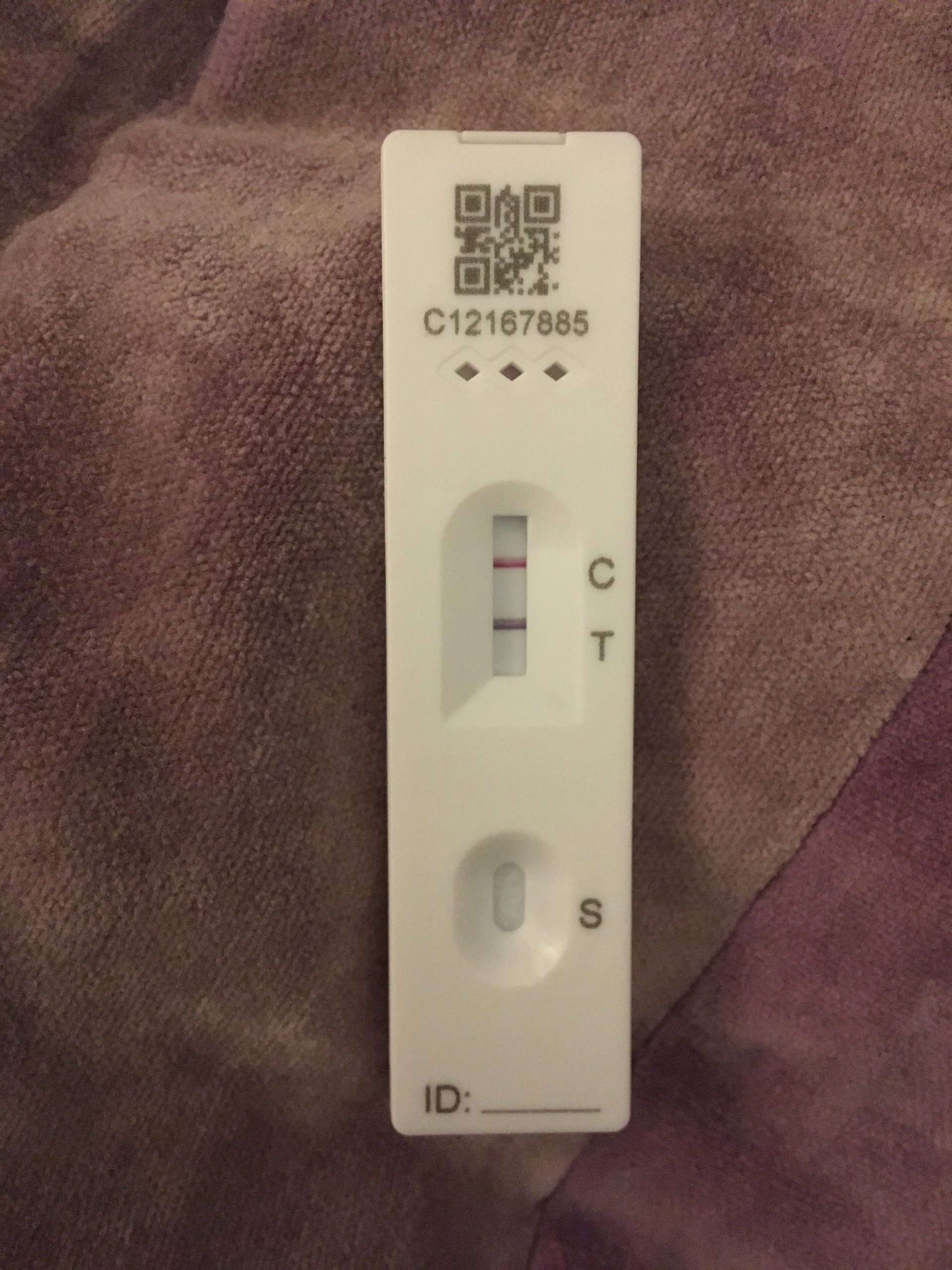
The BinaxNOW™ COVID-19 tests have not been FDA cleared or approved. The BinaxNOW COVID-19 Ag Card 2 Home Test is to be performed only with the supervision of a telehealth proctor. This test is authorized for non-prescription home use with self-collected observed direct anterior nasal (nares) swab samples from individuals aged 15 years or older or adult collected anterior nasal swab samples from individuals aged two years or older. The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal (nares) swabs from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. This test is authorized for use with direct anterior nasal (nares) swab samples from individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested twice over three days with at least 36 hours between tests. The BinaxNOW COVID-19 Ag 2 Card is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal (nares) swab samples from COVID-19 symptomatic individuals tested twice over three days with at least 36 hours between tests within the first seven days of symptom onset. This test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 15 years or older or adult collected anterior nasal swab samples from individuals aged two years or older. The BinaxNOW™ COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. No more lines, no more wait times, no more barriers and no more inconveniences. This combination will help attack the pandemic on critical fronts – speed, simplicity, affordability, access and reliability. You can now access our BinaxNOW test in 3 ways: at your local retailer over the counter self-test, or proctored at-home or from your healthcare professional. Now, with BinaxNOW authorized for over the counter for frequent asymptomatic use, we are making testing directly available for fast results, when and where you need it. The BinaxNOW COVID-19 Self Test card is identical to the professional-use test card, used since August 2020, and is the most studied and widely available rapid antigen test and is now available as a Self Test. ANSWERS, WHEN AND WHERE YOU NEED THEM.Įven while vaccines are rolling out, COVID-19 testing will remain crucial to helping us all return to normal as we begin to engage in everyday life once again.


 0 kommentar(er)
0 kommentar(er)
